Brain-Computer Interfaces: The Latest Breakthroughs and Developments
Last year, we published an article about brain-computer interfaces (BCIs), a rapidly growing sector. Since then, BCIs have continued to capture the spotlight. In 2023, Nature Electronics selected BCIs as the technology of the year, recognizing their critical moment in technological advancement. UNESCO also underscored the importance of this field in various sectors, such as neurostimulation to relieve pain or reduce Parkinson's disease symptoms and improve the daily lives of paralyzed people by releasing a report on neurotechnology.
Synchron and Neuralink are at the forefront of this exciting development, pushing the boundaries of BCI technology with their pioneering approaches to supporting individuals with paralysis. Synchron's COMMAND trial, for example, is a significant step forward. This study focuses on the safety and feasibility of their motor neuroprosthesis (MNP) device, known as the Synchron Switch. Implanted into the brain's blood vessels via a minimally invasive procedure, this device detects and transmits motor intent wirelessly, enabling patients with severe paralysis to control digital devices using their thoughts. Conducted across multiple sites, including Mount Sinai Health System and University at Buffalo Neurosurgery, the COMMAND trial aims to evaluate how the Synchron Switch can assist with daily activities like texting, emailing, and online shopping, thus improving patients' quality of life.
In parallel, Neuralink has initiated its first human clinical trial, the PRIME Study, which aims to assess the safety and efficacy of their BCI technology. Neuralink’s device is designed for direct brain implantation, facilitating high-bandwidth communication between neurons and external devices. Targeting participants with quadriplegia due to cervical spinal cord injury or amyotrophic lateral sclerosis (ALS), this trial will evaluate the device’s potential to restore digital communication and enhance their autonomy.
Both companies are critical in de-risking BCI solutions and bringing them closer to market launch, offering new hope for patients with severe motor impairments. These advancements raise important questions about regulatory, clinical, and market access challenges. To address these, Mass General Brigham, in collaboration with the FDA, launched the iBCI Collaborative Community (iBCI-CC) earlier this year. This initiative brings together researchers, clinicians, medical device manufacturers, patient advocacy groups, and individuals with neurological conditions to advance the field of implantable BCIs (iBCIs). The iBCI-CC aims to propel the field forward through knowledge sharing and interdisciplinary collaboration.
Another example of essential multidisciplinary teamwork in BCI innovation, here in the research field is the NERV team launched by Inria. This team comprises computer scientists, mathematicians, physicists, engineers, neurologists, psychologists, and physiotherapists, all working together to develop algorithms that translate brain activity into commands for controlling external devices like computers or prostheses. These technologies enable users to interact with their environment through thought alone. For stroke patients, the NERV team identifies the brain signals emitted when a patient imagines completing a particular movement, before then using the algorithm to get a muscle prosthetic to carry out the movement. By providing real-time feedback and control over assistive devices, these interfaces aim to accelerate rehabilitation and improve functional abilities, significantly enhancing the quality of life and independence for individuals with paralysis. These collaborative efforts and innovative trials signify a promising future for BCIs, offering new avenues for independence and connectivity for individuals with severe disabilities.
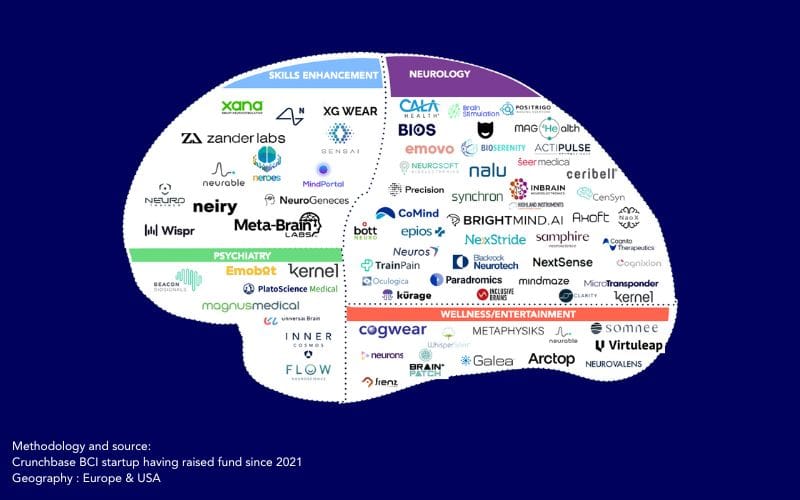
You’ll find below an updated mapping of BCI companies.